


|
Are you looking to
maximize your production potential by
tapping into additional resources and capabilities? At Cytomed Oy, we understand the challenges of finding the right resources to execute your vision. That's why we're offering our underutilized premises, equipment and expertise to support your business needs. We offer you the following contract services and resources for (co)production of medicines, cosmetics or food and feed additives: |
|
 |
Laboratory analysis services: Among the many analytical capabilities of our laboratories, we offer you the following types of analysis:
|
 |
Packaging services: We will be glad to offer you our services of encapsulation and filling of vials with dry powders, blistering, packaging in cardboard boxes with insertion of instructions, serialization, secondary and group packaging, aggregation. |
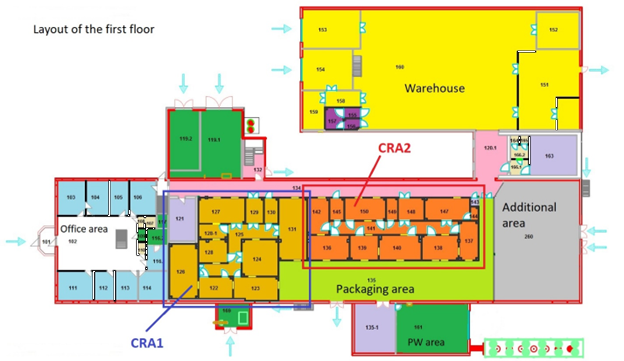 |
Premises: Our pharmaceutical plant has everything necessary for the production of non-sterile dosage forms in accordance with the EU GMP requirements. Two clean room areas are equipped with air and water treatment systems, and the warehouses are air-conditioned. One of the two clean room areas is now completely free and is offered for your use. You are welcome to utilize our premises to house your equipment. |
  |
Equipment: We are ready to offer you our equipment suitable for the following operations of medicines, cosmetics or food and feed additives production:
|
 |
Production of peptides: We have technologies and experience in synthesizing a wide range of high-purity peptides for pharmaceutical, cosmetic, food and feed industries. To launch their full-scale production, joint investments are required. |
|
Production and Support Personnel: Our experienced and highly qualified team is ready to ensure that the (co)production process is set up and running smoothly and in compliance with EU GMP from start to finish. |
|
|
Partnering with us offers several benefits:
We believe that cooperation with us will allow you to open up new opportunities for your business and achieve remarkable results. If you're interested in learning more about us and would like to discuss potential collaboration opportunities, please don't hesitate to reach us out. We look forward to the opportunity to collaborate with your company and contribute to the development of your business. Dmitrii Trutnev, Strategy Planning Manager in International Relations |
|